The law of inertia, referred to as Newton’s first law of motion, states that “an object will remain at rest or in uniform motion in a straight line unless compelled to change its state by the action of an external force”. This is similar to the Coalition for Epidemic Preparedness Innovation’s (CEPI) mission to accelerate the development of vaccines and other biological countermeasures against epidemic and pandemic threats. The traditional vaccine development process can be slow due to factors like regulatory requirements, lengthy clinical trials, and resource limitations. Therefore, progressing from initial research to clinical trials and regulatory approvals may take years. CEPI seeks to overcome this by initiating interventions to accelerate the process and expedite the development of vaccines for diseases like Lassa fever, identified in Nigeria over 50 years ago.
As part of its $3.5 billion plan, CEPI aims to minimise or eliminate future epidemic and pandemic threats by providing support for the development of one or more Lassa vaccines until they are licensed.

Developing a Lassa vaccine
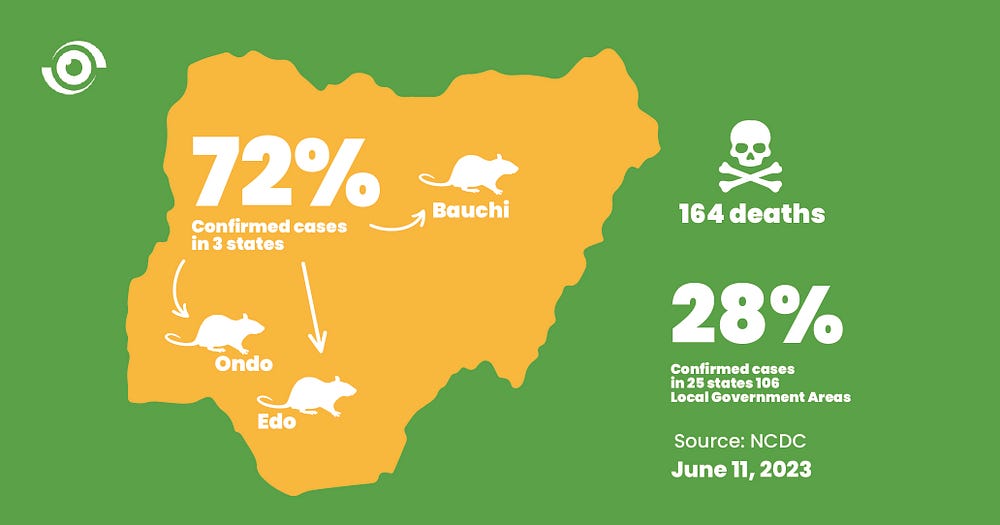
Lassa fever is an endemic disease in Nigeria. As of June 11, 2023, the Nigeria Centre for Disease Control and Prevention (NCDC) had recorded at least one confirmed case of Lassa fever in 28 states, across 106 Local Government Areas (LGAs), with 164 deaths. Seventy-two per cent of all confirmed cases were reported in three states, Ondo, Edo, and Bauchi- while 28% were reported in 25 states.
Under the leadership of the NCDC, the country has developed the capacity to manage outbreaks with improved Lassa fever surveillance, data collection, analysis, and management. However, the goal is to develop a safe, effective, and inclusive vaccine to reduce the burden of Lassa fever on affected populations, enhance public health outcomes, and strengthen global preparedness against future outbreaks.
In an article reflecting on the first-ever Lassa fever International Conference that took place in 2019, Dr Chikwe Ihekweazu, former Director General of the NCDC, said, “While we are yet to begin clinical trials for Lassa fever vaccines in Nigeria, we are much closer than we were a year ago. We are also better involved in discussions with vaccine developers on how to accelerate development of their vaccine candidates. Many of the vaccine developers attended the conference in 2019. This is one area of progress that we are very proud of”.
Accelerating Lassa vaccine development
Lassa fever is listed in the World Health Organization’s (WHO) Research and Development (R&D) list of priority diseases and is one of CEPI’s priority diseases to expedite the development of vaccine candidates. The coalition currently has six Lassa vaccine candidates in its portfolio, four of which have entered the clinical trials phase — Emergent and the International AIDS Vaccine Initiative (IAVI) conducted phase one clinical trials in Ghana and Liberia, respectively. Plans are underway to conduct a ‘Phase IIb’ clinical trial of IAVI’s Lassa fever vaccine among adults and children in Liberia, Nigeria, and Sierra Leone. Results from Enable, the largest Lassa fever research programme being conducted by CEPI in collaboration with authorities in Nigeria, Benin, Guinea, Liberia, and Sierra Leone, will inform future clinical trials.
In June 2023, CEPI and the NCDC organised a workshop with the theme ‘Accelerating Lassa Vaccine Development’ aimed at facilitating collaboration between experts and key stakeholders to expedite the development of a safe and effective Lassa fever vaccine. Federal Government representatives, members of the scientific community, and other stakeholders in attendance shared insights on how to shape the future of Lassa vaccine development in Nigeria. According to Katrin Ramsauer, Lassa fever Programme Manager at CEPI, although the ‘Phase 11b’ study will commence in 2024, it was critical to get the support of these key stakeholders to drive the Lassa fever vaccine development agenda.

Several points emerged from the conversation, including the need to engage with regulatory bodies like the National Agency for Food and Drug Administration and Control (NAFDAC). The need for timely and continuous advocacy to communities to foster trust and collaboration between researchers, healthcare providers, and community members was also discussed. This addresses concerns, allows for open dialogue and incorporates community perspectives into the trial design, implementation, and dissemination of findings. However, for this to be effective, it is important to communicate honestly, with an open mind, and transparently.
According to Dr Nnenaya Ajayi, Case Manager of Lassa Fever at Alex Ekwueme Federal University Teaching Hospital, Abakaliki, “the stage is ripe for a vaccine, and it is good that some work has gone into developing one”. She added that the Lassa fever caseload has been increasing for several years. “Initially, when I got involved with managing Lassa, it wasn’t a yearly affair, but by 2016 it became more like a yearly thing. Initially, it used to happen during the Lassa fever-prone months, but we discovered recently that it is there almost all year round. We also noticed that from the few states that were affected initially, it has spread to many new states”.
The outcome of the conversation resonates with the principles of Newton’s third law, which states that “for every action, there is an equal and opposite reaction”. CEPI invests in vaccine development, coordinates research efforts, and partners with organisations and institutions. Researchers and scientists respond by intensifying their efforts, providing data and insights, and engaging in collaborations. On the other hand, regulatory bodies and policymakers react by evaluating the safety and efficacy of the vaccine candidate. These collective actions and reactions form a collaborative force that propels the vaccine development process forward.
Preparedness, not emergency response
Nigeria can seize this opportunity to strengthen its healthcare infrastructure, yielding long-term advantages for managing Lassa fever and other infectious diseases. By collaborating with international research teams and vaccine developers, the country can enhance local capabilities in crucial areas such as clinical trial management, vaccine development, data analysis, and regulatory compliance. This collaboration fosters sustainable capacity building within the country. Particularly as one of the points raised was the need to ensure local content inclusion in the process to build capacity.
To maximise the advantages, it is important for Nigeria to ensure strong coordination among relevant stakeholders, including government agencies, research institutions, healthcare providers, and communities.
Successful participation in the clinical trials can lead to the development of effective vaccines and countermeasures for Lassa fever. This can have a significant impact on the development of vaccines in Nigeria and beyond, as an effective vaccine will reduce the burden of infectious diseases, save lives, and improve the overall health outcomes of the population.


