By Gabriel Oke and Kenni Ndili (Lead Writers)
In December 2020, the first COVID-19 vaccine was issued emergency use authorisation (EUA) by the US Food and Drug Administration (FDA). This was the Pfizer-BioNTech vaccine, a partnership between a global leader in vaccine manufacturing and a German biotechnology company. Vaccine campaigns slowly began to take place across the world as countries began to vaccinate their populations. Through the vaccine alliance, COVAX, a partnership between the Coalition for Epidemic Preparedness Innovations (CEPI), Gavi, United Nations Children’s Fund (UNICEF) and the World Health Organisation (WHO), African countries began receiving vaccine allocations after a negotiation process took place to determine the number of vaccines each country could receive.

On March 2, 2021, Nigeria received 3.94 million doses of the AstraZeneca COVID-19 vaccine, shortly after Ghana received theirs in late February. The arrival of the vaccines enabled the National Primary Health Care Development Agency (NPHCDA) to commence the vaccination of Nigerians, adopting the national vaccine deployment plan (NDVP) alongside the advisory to start a vaccine campaign targeting frontline health workers, the elderly, and members of vulnerable populations. As of August 9, 2021, about 1.4 million people have been fully vaccinated, with 2.5 million receiving their first dose. With the arrival of the second round of vaccines on August 11 and recent shipment of the single-shot Johnson & Johnson vaccines, it is important to assess the first rollout process as the nation prepares for the second rollout.
Vaccine Rollout Stages
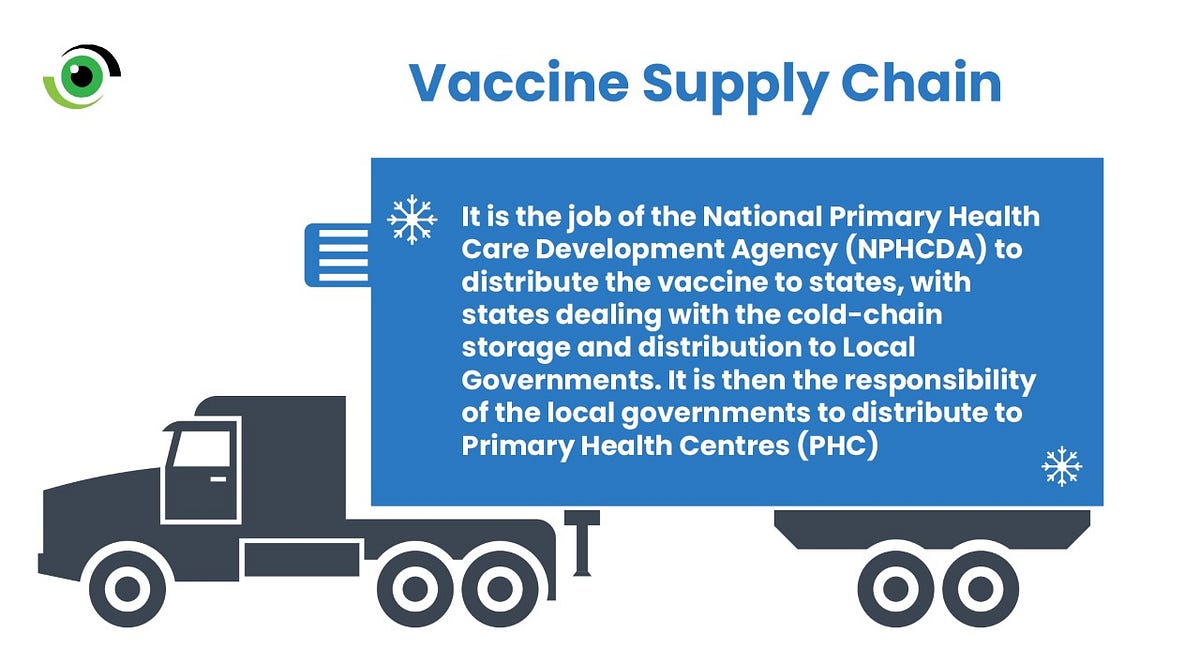
The ability to effectively rollout the vaccine lies in the strength of the vaccine supply chain. It is the job of the NPHCDA to distribute the vaccine to states, with states dealing with the cold-chain storage and distribution to local governments. It is then the responsibility of the local governments to distribute to Primary Health Centres (PHC). To make it easy for states to roll out their vaccine doses, the NPHCDA partnered with agencies, including WHO and UNICEF to deploy electronic self-registration and house-to-house registration for the vaccination campaign across the 36 states and Federal Capital Territory. “A lot of demand generation and sensitisation of critical stakeholders have gone into the preparedness towards the vaccine rollout”, said Dr Faisal Shuaib, Executive Director of the NPHCDA at a program organised to launch the rollout of the first doses.
However, though transportation from national to state stores has been relatively reliable, there have been some supply chain breakdowns from local cold-stores to end-points and glitches in logistics and deployment across states. Oyo State for example experienced power difficulties in many of its storage facilities at the beginning of the rollout of the first vaccine doses.
Ekiti State Commissioner for Health speaking in an interview about the first rollout said, “We thought the federal government would oversee logistics through the Ministries and Development Agencies and support mobilisation at state level”. The state had to come up with a plan immediately; engage new people and make provision for data.”

Vaccine Rollout: Encouraging Uptake
Misinformation surrounding the COVID-19 vaccines was a challenge that states had to tackle instantly. Due to the swift nature of the vaccine’s development and deployment, there had been some hesitancy around receiving the vaccine. To encourage vaccine uptake at national level, President Muhammadu Buhari and Vice-President Yemi Osinbajo received the COVID-19 vaccine publicly. Kayode Fayemi, Chairman of the Nigerian Governors’ Forum, also disclosed that governors of the 36 states of the federation and their deputies would take the vaccine to encourage their state population to take the vaccine. On 12 March 2021, states across Nigeria began to distribute and administer COVID-19 vaccines, with priority being given to frontline health workers, laboratory staff, covid-19 Rapid Response Team, the police, and strategic leaders.

To increase uptake at state level, Ekiti state organised a ceremony at the beginning of the roll out where the state governor and political, union and religious leaders in the state were vaccinated publicly. Risk communication was done using social media, electronic media, and town hall meetings. The state also started a social media campaign encouraging vaccine takers to post on their social media accounts and providing photo booths to encourage more vaccine uptake.
Ekiti State also set up a Technical Working Group (TWG) made up of the Permanent Secretary of the State Ministry of Health, State Immunisation officer, Permanent Secretary, Hospital Management Board, State Epidemiologist, Members of the Emergency Operation Centre for COVID-19, Senior Special Adviser to the State Governor on Public Health and the State cold chain officer. The group met every day to mobilise at the state level. The TWG then involved everyone at the Ministry of Health to be able to course correct problems using daily performance management frameworks. Thus, the state made daily improvements to their implementation process.
In the first week of rollout in Kano State, state authorities had to combat a lack of public confidence. Through the first few weeks of the vaccination there were many sceptics, with health workers also expressing apprehension about the vaccine. However, a group of people came forward willingly to receive the jab for reasons like they have “old parents,” or “sick husbands/relatives” who they did not want to infect with the virus. In addition, the state utilised local media stations to enlighten the public about the importance of the vaccine, simultaneously repelling fake news aimed at discrediting its efficacy and increasing public confidence in the vaccine.
In February 2021, Nigeria Health Watch conducted a COVID-19 Household Vaccine survey and religious beliefs showed up strongly as a reason why people were unwilling to take the vaccine. To increase vaccine acceptance and uptake, religious leaders and other traditional leaders should be educated and encouraged to pass the right messages and address concerns about the vaccine at the grassroots.

To fight against apprehension surrounding the vaccine, states like Lagos enacted robust surveillance to monitor and track adverse events experienced by vaccine recipients like pain at the injection site, fever or body pains lasting 24 to 48 hours, while those experiencing symptoms were advised to contact their LGA Disease Surveillance and Notification structures for continuous monitoring. They also conducted active risk communications to manage the expectation of the recipients and educate them on proper monitoring of adverse events and how to inform official channels.
Leveraging on Public Private Partnerships
With the Nigerian government set to receive another 4 million vaccine doses in August 2021, the government must look into ways to make distribution more efficient and encourage uptake.

To ensure vaccine rollout is undertaken effectively, it is important that the vaccine supply-chain is strengthened between local governments and PHCs, so vaccines are stored and dispatched securely. Dr Pamela Ajayi, the president of the Healthcare Federation of Nigeria (HFN), believes that the private sector can play a pivotal role in the effective rollout of the second round. “The private sector is ready to work with the government across the entire value chain, from storage to distribution and administration. Many states have limited funding to facilitate the cost of administration and need support in areas like human resources, logistics, cold chain management, electricity and security to mention a few.”
In order to create a public-private partnership model that will aid rollout, she highlights the Lagos Biobank model as a case study. “An ideal model to use would be one like the Lagos State Biobank model used for Covid-19 testing. There is a system which tests the centres before they are accredited, with training to ensure they adhere to Lagos State Biobank protocols and guidelines. It is strictly regulated to ensure high standards and fast turnaround times. It has been useful in creating many jobs and enabled the opening up of the economy.”
The COVID-19 vaccine is the fastest and safest way to combat the COVID-19 virus. Public-private partnerships may be the most effective way to ensure that the vaccine rollout is performed safely and effectively, providing critical support to the government where necessary. Therefore, as the country prepares to rollout the next batch of vaccines from the 16th August, states should learn from the challenges experienced during the first rollout, making every effort to address them and seek avenues for collaboration and partnership, taking note of opportunities to improve and make the distribution more efficient in order to encourage greater uptake of COVID-19 vaccines.



In my own opinion, I think the aftereffect of the vaccine has dissuaded so many people from getting vaccinated. Notably, people tend to exaggerate the aftereffects.